Non-equilibrium biomolecular condensates
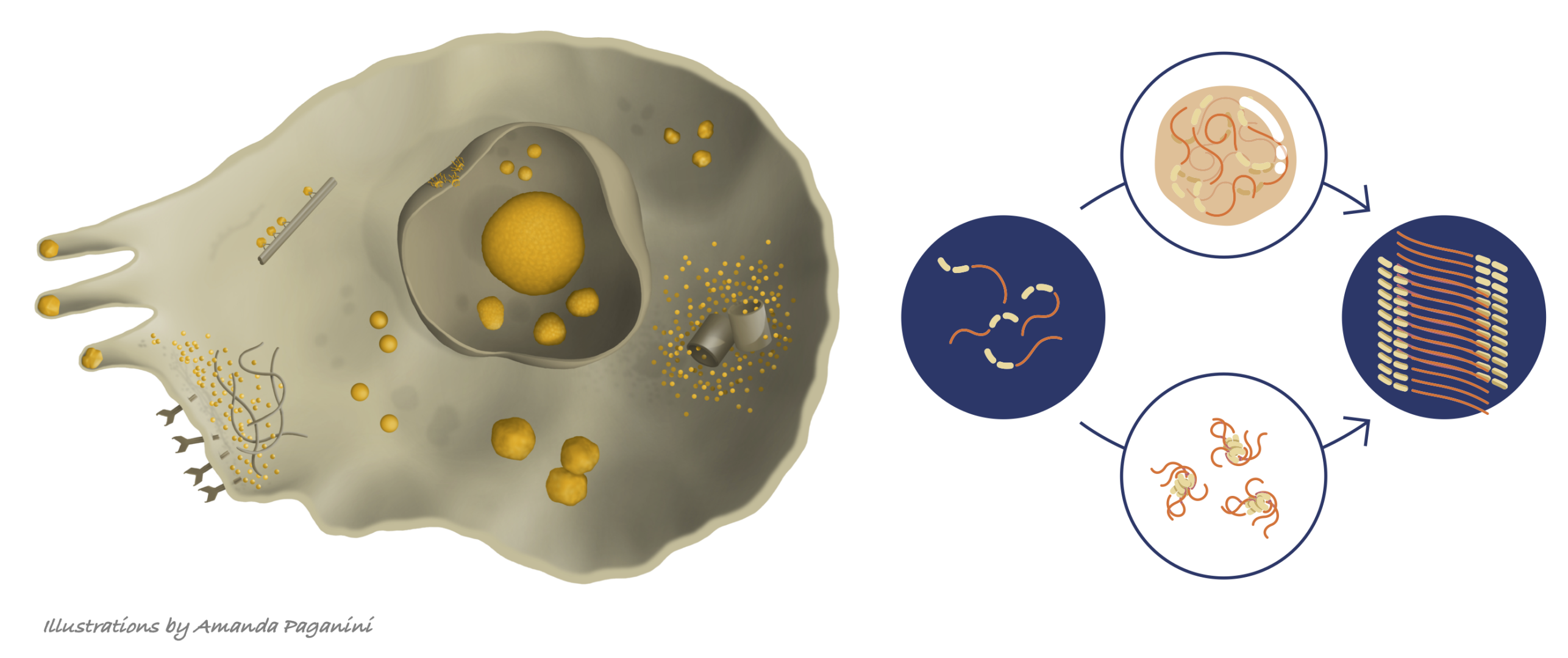
Self-assembly of biomolecules is crucial in both functional and dysfunctional biology. Protein aggregation and phase transition are increasingly associated with regulation of cellular functions in space and time, as well as with pathological conditions including Alzheimer’s and Parkinson’s disease. Since biological systems are out-of-equilibrium, in addition to thermodynamics, kinetics is crucial to govern the behavior of the system. Proteins can access a variety of physical states, ranging from dynamic condensates to irreversible aggregates, which may be only metastable and under kinetic control. We apply traditional and modern tools of chemical reaction engineering, including microfluidics, to characterize the molecular determinants of the dynamics of condensates, focusing on two major problems: i) maturation of condensates into amyloid fibrils, and ii) material properties and aging of condensates of DEAD-box RNA binding proteins associated with cell compartmentalization and RNA metabolism.
Contacts
Condensates and amyloids: Chiara, Marcell, Lenka
Condensates and material properties: Charlotte, Timo, Kasia
Key publications